Determining Phage Titer
Materials
- Phage lysate or freezer stock (Procedure: Phage Lysate Preparation)
- Top Agar (LB-Miller + 0.8% agar)
- LB-MIller (for diluting phage)
- Small test tubes
- Heat block @ 55°C that can hold individual small test tubes
Procedure
- Microwave flask of Top Agar to melt. Allow to cool until handleable, then prepare 4 ml aliquots in small test tubes for as many samples as you will be handling. Place tubes in a heat block or water bath set at 55-60°C.
- Prepare dilutions of the phage stock in LB-Miller. Typically a dilution series from 100; to 1010 in 102 increments will give one plate with a countable number of plaques. These dilutions can be easily prepared by making 1.7 ml Eppendorf tubes with 990 µl LB and transferring 10 µl from the higher stock, mixing, and transferring to the next sample.
- Combine 100 µl of exponentially growing host cells and 100 µl of phage dilution in a fresh Eppendorf.
- Optional: Incubate unshaken at 37°C for 30 minutes.
- Add each sample to a top agar aliquot. Quickly mix by pipetting up and down or gently vortexing. Avoid bubbles. Pour the entire contents of test tube on top of an LB plate and rapidly rotate the plate around so that the agar covers the entire surface evenly before it cools.
- Let the plates cool ~15-30 minutes at room temperature and then transfer to an incubator at 30°C or 37°C.
Expected Results
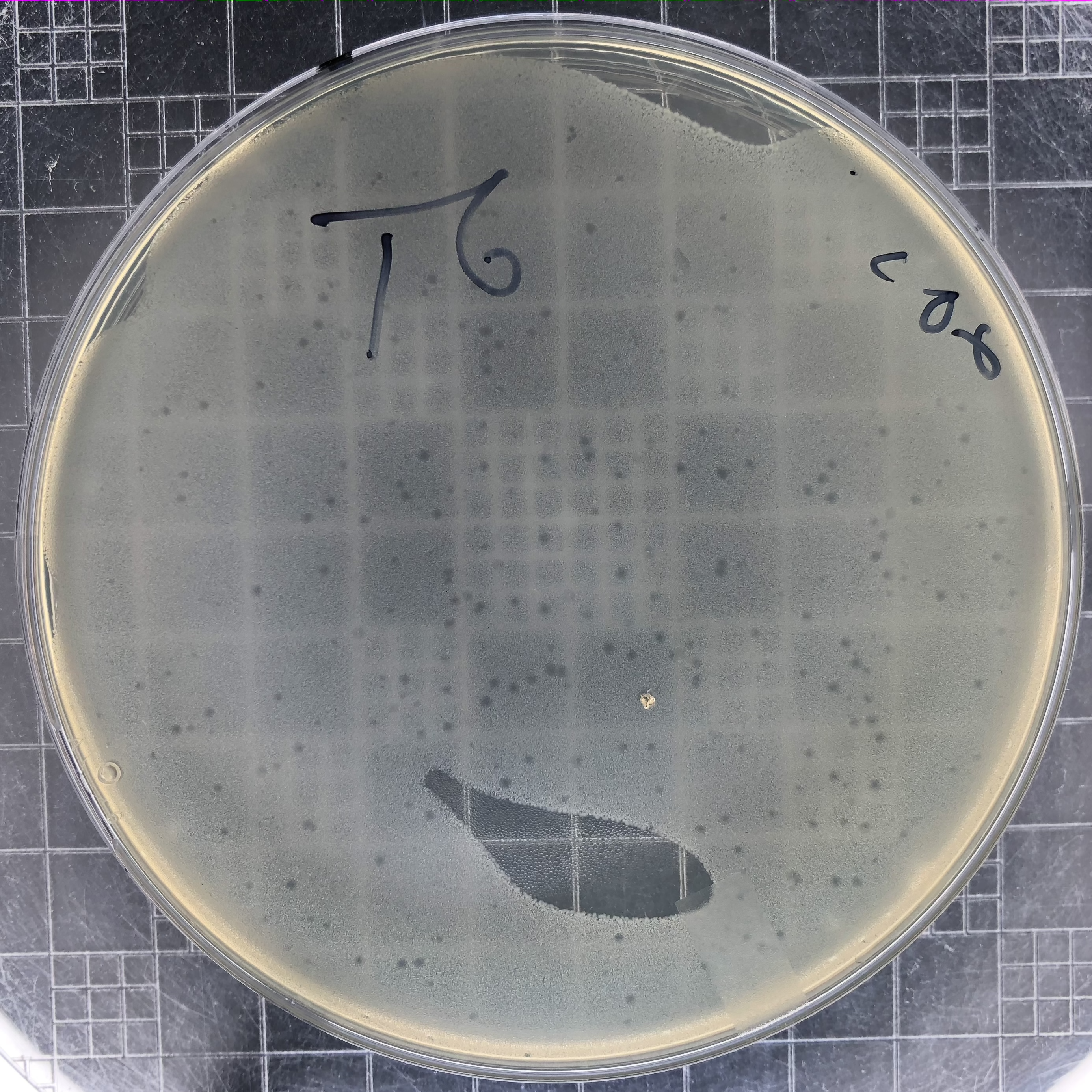 |
Phage T6 plaques after overnight growth at 37°C of a 106 dilution of lysate. Plaques for most E. coli T-even phages resemble these. They reach a maximum size and then stop spreading. |
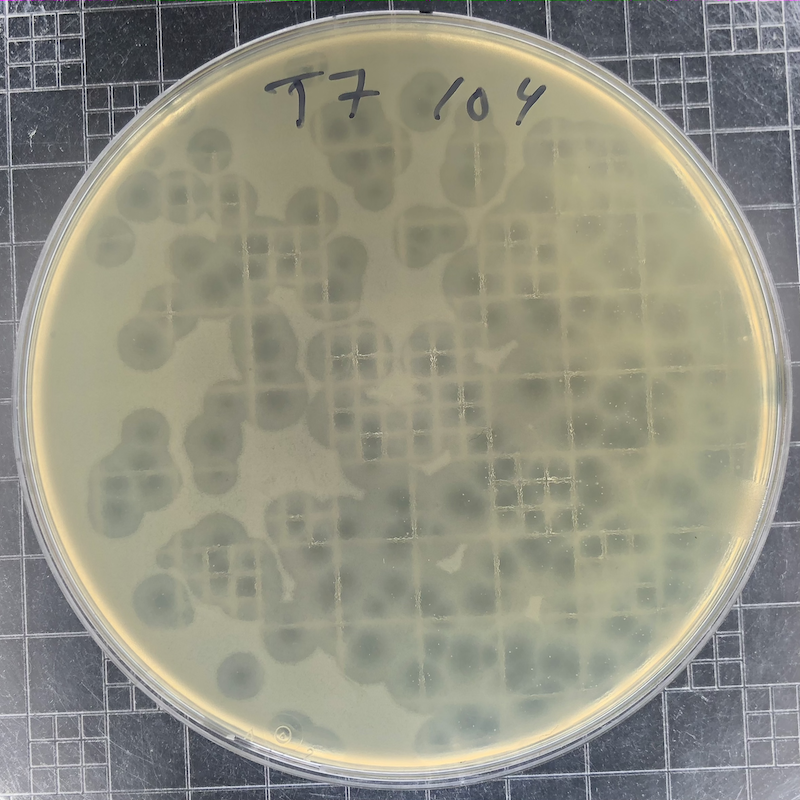 |
Phage T7 plaques after overnight growth at 37°C of a 104 dilution of a freezer stock. T7 plaques continue to grow. Incubate the plates at 30°C if you want to slow plaque growth. |
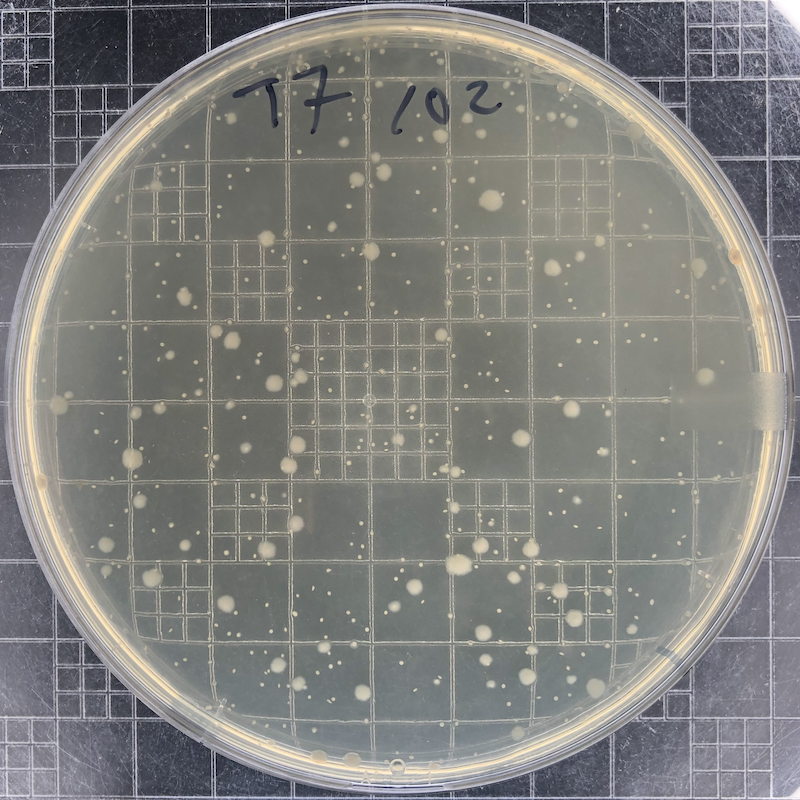 |
Phage T7 plaques after overnight growth at 37°C of a 102 dilution of a freezer stock. The phage has lysed all bacteria in this plate. The remaining colonies (small if they are embedded in the top agar and large if they reach the surface) are derived from mutant bacterial cells that have become resistant to the phage. |
Example of Calculating Phage Titer
Since you are plating 100 µl of each stock you should multiply by 10 and also by the dilution of the tube that gave a countable number of plaques to determine the PFU/ml in the original lysate. So, if there were 25 plaques in the 100 µl you mixed in of the 106 dilution, then the lysate would have 2.5×108 PFU/ml.| I | Attachment | History | Action | Size | Date | Who | Comment |
|---|---|---|---|---|---|---|---|
| |
T6-plaques-1E6.png | r1 | manage | 8914.4 K | 2020-02-24 - 19:37 | JeffreyBarrick | |
| |
T7-plaques-1E2.png | r1 | manage | 1079.4 K | 2020-02-24 - 19:04 | JeffreyBarrick | |
| |
T7-plaques-1E4.png | r1 | manage | 989.0 K | 2020-02-24 - 19:03 | JeffreyBarrick |
Barrick Lab > ProtocolList > ProtocolsPhageAdsorptionRate > ProtocolPhageLysate > ProtocolsPhageTiters
Topic revision: r3 - 2020-02-24 - 19:42:22 - Main.JeffreyBarrick

