Freezing Strains
Supplies
- Overnight liquid culture
Several milliliters of overnight liquid culture grown in a suitable medium for each sample to be frozen. - 80% Glycerol or DMSO solution
A standard lab solution. Grab your own stock bottle and keep it in your workspace. - Sterile 2 ml cryovials
Take a bag for yourself and keep it in your workspace, closing it with an alligator clip when not in use. - Cryotag rectangular 1.28" x 0.50" labels
Found in the office supplies. - Clear tape
Found in dispensers throughout lab. Do NOT use "magic" tape (which is translucent and does not stick as well at –80°C). Use fully clear tape. - Freezer box
With 81 tube insert (9 × 9 grid).
Labeling Cryovials
Materials for labeling a cryovial and closeup of properly labeled vial
- Sample naming – Every strain has a three letter designation (usually the initials of someone) and a strain number. When freezing new samples, either append them to your own strain series or obtain the next available numbers from a standard series (e.g. JEB) by looking in the Strain Database.
- Write the three letter designation, the strain number, and the date (MM–DD–YY) on a CRYOTAG label for each sample. Also include whatever other distinctive information about the strain that can be fit onto the label (e.g. experiment, population, time).
- Stick the label on tube horizontally, and completely cover it with one strip of clear tape for longevity.
- Write the strain number on the plastic circular cap insert, so that it will be visible from above when looking down on the tube in a freezer box.
Labeling Freezer Boxes
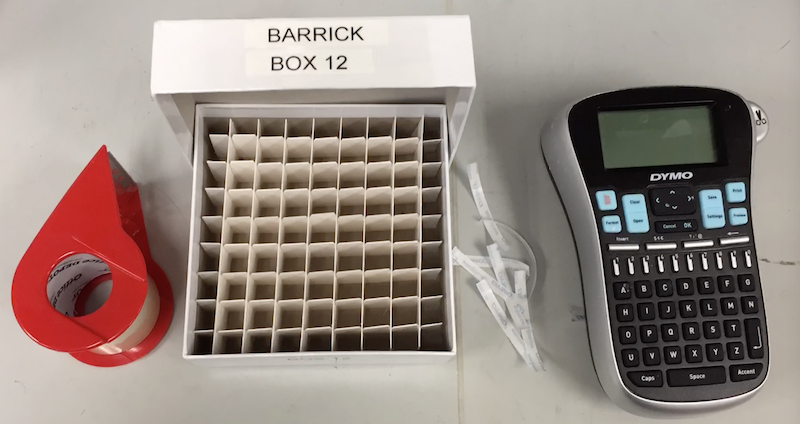
Example of a labeled freezer box
- Box Naming - Every box should be labeled with your last name and a number. Use this system and not a special name for each box.
- Print out two copies of your labels with the Dymo labeler. One should have your last name and one should have "Box X" on it.
- Place the labels on both the bottom and lid of your box. Do this on a dry and room-temperature box so that they will stick. Do not do it on one that you have already had in the freezer!
- Completely cover labels in clear packing tape for longevity.
Freezing Samples
You will need to add a cryoprotectant to your sample of cells to be frozen. Generally this is 80% glycerol, though for some experiments DMSO is used instead. The standard amount to add of this cryoprotectant is one-fifth the volume of your cells. This gives a final concentration of 13.3% glycerol or 16.7% DMSO. If you are using DMSO, wear gloves and avoid contact with your skin! For most samples, you can follow this procedure: 1 Add 200 µl of 80% glycerol to each empty cryovial. Glycerol is extremely viscous. Use the 1 ml pipettor because the tips have a larger aperture. Be careful to pull liquid into the pipette tip slowly and to let excess liquid drip off the outside before you remove the tip from the stock bottle. Use the repeat pipettor if you are freezing >12 samples.- Add 1000 µl of each revived strain to the appropriately labeled cryovial using a 1 ml pipettor.
- Vortex each cryovial at high speed to be sure that these solutions are uniformly mixed.
- Place the tubes in the next positions available in your boxes or the main lab stock boxes. Record the box, rack, and freezer names and numbers.
Updating the Strain Database
It is very important to immediately add information about the samples you have archived to the lab database so that they can be located by others and posterity. You should also keep a record in your lab notebook of where these samples came from. Instructions for how to use the strain database are located here:Additional information for strains from ATCC
Strains from ATCC typically arrive as freeze-dried samples. After you break the vial, you should resuspend the cells in a ~400 µl of the media suggested by ATCC for growth of your strain. Because these are type strains and we don't want to accidentally select a mutant as our version of the type strain, we archive the following samples:- Mixed Population: Immediately add ~100 µl of the resuspension (~1/4 of the total volume) to liquid media. This outgrowth will be the original "mixed population" sample.
- Clonal Isolates: Streak out from the remainder of the resuspension on a suitable agar plate. Grow as directed, pick 3 colonies to grow in liquid media, and archive as c1, c2, c3. These are the "clonal isolate" samples. The c1 sample will be the one that you use in your experiments and check genetically and phenotypically to be sure it is the correct strain, and not a mutant or contamination!
- Freeze-Dried: Freeze the remainder of the original resuspension of the freeze-dried culture at –80°C using 10-20% DMSO as cryoprotectant. This is the "original freeze-dried stock".
Barrick Lab > ProtocolList > ProtocolsFreezingStrains
Topic revision: r7 - 2016-12-15 - 14:46:18 - Main.JeffreyBarrick