<<Return to qPCR page
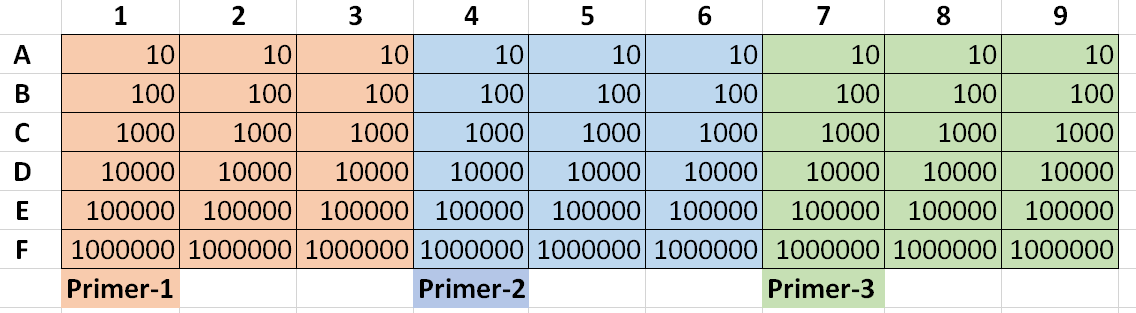 (Numbers are PCR product dilution with "10" representing a 1:10 dilution of your 0.01ng/ul PCR product prepped as described above)
Template Material
(Numbers are PCR product dilution with "10" representing a 1:10 dilution of your 0.01ng/ul PCR product prepped as described above)
Template Material
Primer Efficiency qPCR
THIS PAGE IS CURRENTLY UNDER CONSTRUCTION Goals- Test that primers work
- Determine the efficiency with which your primers bind to your target (This will be important when you do your final analysis)
- The template for this qPCR will be the PCR product of each of your target genes. So before you get started you will need to perform PCR off of either genomic DNA or cDNA (this depends on how you designed your primers)
- Run your PCRs like you normally would (use a 60C annealing temperature).
- PCR purify your product and then dilute to a concentration of ~ 0.01ng/ul (trust me...it seems low but it works!)
- Now you're good to go ahead and set up your qPCR reactions!
 (Numbers are PCR product dilution with "10" representing a 1:10 dilution of your 0.01ng/ul PCR product prepped as described above)
Template Material
(Numbers are PCR product dilution with "10" representing a 1:10 dilution of your 0.01ng/ul PCR product prepped as described above)
Template Material
- Use PCR product as your template for this one!
- Technical replicates with a standard deviation below 0.2 (this is especially important for this step because when your standard deviations are high you'll calculate inaccurate primer efficiencies - this qPCR step is thus nice to do first because it's good practice for getting those SDs low without wasting your precious biological samples)
- Ct values for the entire range of these dilutions. If any of the extremes (1:10 or 1:100,000) are "Undetermined" you may want to increase or decrease the dilution of your PCR template (I recommended an initial concentration of 0.01ng/ul, but if this does not work for you adjust it as needed!). You need to have a range of values that stretch over 6 10-fold dilutions to get a good calculation of primer efficiency. It's also ideal that all the Ct values fall within the dynamic range of 13-30; sometimes values that fall out of that range will skew your data and mess with your efficiency calculation.
Barrick Lab > ProtocolList > QPCR > PrimerEfficiencyqPCR
Contributors to this topic  KateElston, DanielDeatherage
KateElston, DanielDeatherage
Topic revision: r4 - 2020-02-20 - 21:19:43 - Main.KateElston

 Mol Biosciences
Mol Biosciences The LTEE
The LTEE iGEM team
iGEM team NGS course
NGS course