
Difference: MEGAWHOP (1 vs. 12)
Revision 122018-10-25 - SeanLeonard
Megaprimer whole plasmid cloning | |||||||||
| Changed: | |||||||||
| < < | aka MEGAWHOP cloning aka Overlap Extension PCR cloning | ||||||||
| > > | aka MEGAWHOP cloning aka Overlap Extension PCR cloning | ||||||||
| Added: | |||||||||
| > > | We describe two approaches to MEGAWHOP in this protocol page. | ||||||||
| Added: | |||||||||
| > > | Approach 1: | ||||||||
Adapted from Bryksin AV, Matsumura I. 2010. Overlap extension PCR Cloning: a simple and reliable way to create recombinant plasmids. Biotechniques.48(6):463-5.
PurposeTo insert a DNA sequence into a plasmid without restriction enzymes.Experimental Steps
Designing Primers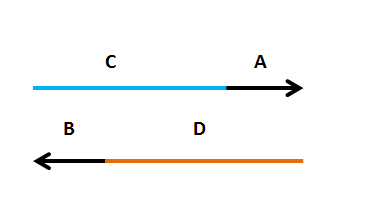 Primers need to have two components
Primers need to have two components
PCR InsertUse standard 25ul Phusion (or other high fidelity polymerase) protocol
PCR Recombinant PlasmidUse modified 10ul Phusion (or other high fidelity polymerase) protocol
DigestOnce the reaction is complete, digest the Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA. DpnI DigestTransformHeatshock 5ul of the Dpn1-digested MEGAWHOP reaction mixture into 25ul of chemically competent E coli.ExampleChange the promoter for sgRNA using MegaWHOP. Template sequence: https://benchling.com/s/g4S95i24 Primers:
 *PCR Recombinant Plasmid:
*PCR Recombinant Plasmid: 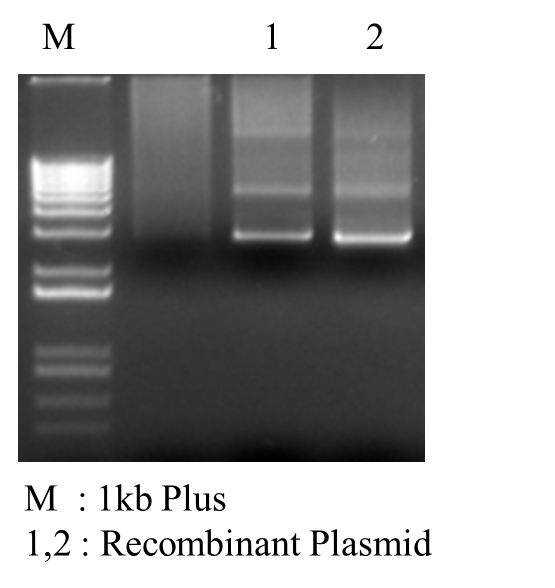 | |||||||||
| Added: | |||||||||
| > > |
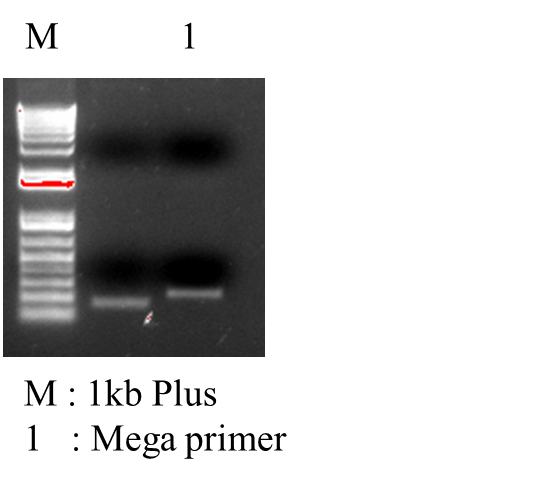
Approach 2:IntroductionThis protocol was inspired (after failed site directed mutagenesis attempts using Quikchange) by the protocol listed here at the Colgate website here. There are several other method papers that describe this (or variant) MEGAPRIMER and MEGAWHOP protocols, there is one here and the original here.This technique is useful for introducing single base pair, single amino acid, or multiple changes into a gene residing on a plasmid that you already have. In short, one mutagenic primer is paired with a standard primer to create a “Megaprimer” product that contains your mutation(s) of choice, is 200-400 bp long, and contains at least >=15 bp of homology on each end to your template plasmid. (You could, of course, just order a gene block with of this size containing all of your desired mutations and use it as a megaprimer). This megaprimer is purified and then used as primer with the template plasmid in a second PCR whole plasmid (Megawhop) reaction. This PCR product is cleaned up and transformed into competent cells to yield your desired mutation. Required MaterialsTemplate PlasmidCompetent Cells PCR Reagents Culturing Materials (>= 4 plates with appropriate antibiotics for transformations) Sequence of desired mutation Primer Upstream of Desired mutation Procedure1. Design a mutagenic primer with your desired mutation. Ensure at least 15 base pairs of homology downstream of your mutation.2. Create your megaprimer by running PCR using your upstream primer and designed primer with a standard Phusion protocol. Your template is your unmutated gene product. 2x 50 uL reactions are sufficient. This megaprimer will contain your desired mutations. These steps have worked:
4. Use this purified megaprimer product and your template plasmid in a 2nd PCR (2x 50 uL reactions). Your control in this reaction is a reaction without your megaprimer from the previous step. Use commercial Phusion for this reaction (or other super high fidelity polymerase). Each tube should contain very little template plasmid (1 uL or 50 ng) and 5 uL of your purified megaprimer product. Concentrations for the rest of your PCR reagents are standard. These steps have worked:
6. Optional You can run 5uL of your completed PCR reaction on an agarose gel and compare it to (1) your control reaction and (2)an unreacted mixture of the PCR components. You should see a faint band at your expected plasmid size only in your primer-containing reaction (DpnI should digest the template in both reactions). 7. PCR Cleanup both your sample and control reactions. 8. Transform 1-2uL of each reaction into competent cells using standard electroporation or chemical transformation protocols. 9. Plate dilutions of both reactions on appropriate antibiotic plates for selection. 10. If your sample shows more colonies than your control, the process was likely successful. Pick 3 clones to make stocks and send for sequencing (link) | ||||||||
| |||||||||
Revision 112017-12-14 - PengGeng
Megaprimer whole plasmid cloning | |||||||||
| Changed: | |||||||||
| < < | aka MEGAWHOP cloning aka Overlap Extension PCR cloning | ||||||||
| > > | aka MEGAWHOP cloning aka Overlap Extension PCR cloning | ||||||||
Adapted from Bryksin AV, Matsumura I. 2010. Overlap extension PCR Cloning: a simple and reliable way to create recombinant plasmids. Biotechniques.48(6):463-5.
Purpose | |||||||||
| Deleted: | |||||||||
| < < | To insert a DNA sequence into a plasmid without restriction enzymes. | ||||||||
| Added: | |||||||||
| > > | To insert a DNA sequence into a plasmid without restriction enzymes. | ||||||||
Experimental Steps
Designing Primers Primers need to have two components
Primers need to have two components
PCR Insert | |||||||||
| Changed: | |||||||||
| < < | Use standard 25ul Phusion (or other high fidelity polymerase) protocol | ||||||||
| > > | Use standard 25ul Phusion (or other high fidelity polymerase) protocol | ||||||||
PCR Recombinant Plasmid | |||||||||
| Changed: | |||||||||
| < < | Use modified 10ul Phusion (or other high fidelity polymerase) protocol | ||||||||
| > > | Use modified 10ul Phusion (or other high fidelity polymerase) protocol | ||||||||
Digest | |||||||||
| Changed: | |||||||||
| < < | Once the reaction is complete, digest the Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA. DpnI Digest | ||||||||
| > > | Once the reaction is complete, digest the Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA. DpnI Digest | ||||||||
Transform | |||||||||
| Changed: | |||||||||
| < < | Heatshock 5ul of the Dpn1-digested MEGAWHOP reaction mixture into 25ul of chemically competent E coli. | ||||||||
| > > | Heatshock 5ul of the Dpn1-digested MEGAWHOP reaction mixture into 25ul of chemically competent E coli. | ||||||||
Example | |||||||||
| Changed: | |||||||||
| < < | Change the promoter for sgRNA using MegaWHOP. | ||||||||
| > > | Change the promoter for sgRNA using MegaWHOP. | ||||||||
Template sequence: https://benchling.com/s/g4S95i24
Primers:
 *PCR Recombinant Plasmid:
*PCR Recombinant Plasmid: 
| |||||||||
Revision 102017-06-13 - GabrielSuarez
Megaprimer whole plasmid cloning | |||||||||
| Changed: | |||||||||
| < < | aka MEGAWHOP cloning | ||||||||
| > > | aka MEGAWHOP cloning aka Overlap Extension PCR cloning | ||||||||
| Deleted: | |||||||||
| < < | aka Overlap Extension PCR cloning | ||||||||
Adapted from Bryksin AV, Matsumura I. 2010. Overlap extension PCR Cloning: a simple and reliable way to create recombinant plasmids. Biotechniques.48(6):463-5.
PurposeTo insert a DNA sequence into a plasmid without restriction enzymes.Experimental Steps
| |||||||||
| Changed: | |||||||||
| < < | | ||||||||
| > > | |||||||||
| Deleted: | |||||||||
| < < | |||||||||
Designing Primers | |||||||||
| Changed: | |||||||||
| < < |  | ||||||||
| > > |  | ||||||||
Primers need to have two components
PCR Insert | |||||||||
| Changed: | |||||||||
| < < | Use stardard 25ul Phusion (or other high fidelity polymerase) protocol | ||||||||
| > > | Use standard 25ul Phusion (or other high fidelity polymerase) protocol | ||||||||
| |||||||||
| Changed: | |||||||||
| < < |
| ||||||||
| > > |
| ||||||||
| |||||||||
| Changed: | |||||||||
| < < |
| ||||||||
| > > |
| ||||||||
| |||||||||
| Changed: | |||||||||
| < < | Purify PCR products. This can either be done through a gel extraction or by adding Dpn1 directly to the Phusion reaction mixture after PCR, digesting at 37°C for 1 hour, and then doing a standard PCR clean up. Dpn1 works efficiently in a Phusion reaction mixture. | ||||||||
| > > | Purify PCR products. This can either be done through a gel extraction or by adding Dpn1 directly to the Phusion reaction mixture after PCR, digesting at 37°C for 1 hour, and then doing a standard PCR clean up. Dpn1 works efficiently in a Phusion reaction mixture. | ||||||||
PCR Recombinant Plasmid | |||||||||
| Changed: | |||||||||
| < < | Use modified 10ul Phusion (or other high fidelity polymerase) protocol | ||||||||
| > > | Use modified 10ul Phusion (or other high fidelity polymerase) protocol | ||||||||
| |||||||||
| Changed: | |||||||||
| < < |
| ||||||||
| > > |
| ||||||||
| |||||||||
| Changed: | |||||||||
| < < |
| ||||||||
| > > |
| ||||||||
| |||||||||
| Changed: | |||||||||
| < < | Adjust Elongation time for the length of the entire plasmid (90 seconds per kb). | ||||||||
| > > | Adjust Elongation time for the length of the entire plasmid (90 seconds per kb). | ||||||||
| OR | |||||||||
| Changed: | |||||||||
| < < | |||||||||
| > > | Use modified 25ul Phusion protocol | ||||||||
| Deleted: | |||||||||
| < < | Use modified 25ul Phusion protocol | ||||||||
| |||||||||
| Changed: | |||||||||
| < < |
| ||||||||
| > > |
| ||||||||
| |||||||||
| Changed: | |||||||||
| < < | 68°C 5min+98°C 3min+(98°C 30s+68°C 30s+72°C X min)*30 +72°C 10min | ||||||||
| > > | 68°C 5min+98°C 3min+(98°C 30s+68°C 30s+72°C X min)*30 +72°C 10min Adjust Elongation time for the length of the entire plasmid (30 seconds per kb). | ||||||||
| Deleted: | |||||||||
| < < | Adjust Elongation time for the length of the entire plasmid (30 seconds per kb). | ||||||||
| Changed: | |||||||||
| < < | DigestOnce the reaction is complete, digest the Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA. DpnI Digest | ||||||||
| > > | DigestOnce the reaction is complete, digest the Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA. DpnI Digest | ||||||||
TransformHeatshock 5ul of the Dpn1-digested MEGAWHOP reaction mixture into 25ul of chemically competent E coli. | |||||||||
| Deleted: | |||||||||
| < < | |||||||||
ExampleChange the promoter for sgRNA using MegaWHOP. Template sequence: https://benchling.com/s/g4S95i24 | |||||||||
| Changed: | |||||||||
| < < | Primers: | ||||||||
| > > | Primers: | ||||||||
| |||||||||
| Changed: | |||||||||
| < < |
| ||||||||
| > > |
| ||||||||
| UPCASE LETTERS: a region that amplifies the insert (A(or B) | |||||||||
| Changed: | |||||||||
| < < | lower case letters: a region that targets the new plasmid (C(or D) | ||||||||
| > > | lower case letters: a region that targets the new plasmid (C(or D) | ||||||||
| Changed: | |||||||||
| < < | *PCR MEGA_primer: | ||||||||
| > > | *PCR MEGA_primer:  | ||||||||
| Deleted: | |||||||||
| < < |  | ||||||||
| Changed: | |||||||||
| < < | *PCR Recombinant Plasmid: | ||||||||
| > > | *PCR Recombinant Plasmid:  | ||||||||
| Deleted: | |||||||||
| < < | 
| ||||||||
| |||||||||
Revision 92016-12-15 - PengGeng
Megaprimer whole plasmid cloningaka MEGAWHOP cloningaka Overlap Extension PCR cloning Adapted from Bryksin AV, Matsumura I. 2010. Overlap extension PCR Cloning: a simple and reliable way to create recombinant plasmids. Biotechniques.48(6):463-5. PurposeTo insert a DNA sequence into a plasmid without restriction enzymes.Experimental Steps
Designing Primers Primers need to have two components
Primers need to have two components
PCR InsertUse stardard 25ul Phusion (or other high fidelity polymerase) protocol
PCR Recombinant PlasmidUse modified 10ul Phusion (or other high fidelity polymerase) protocol
DigestOnce the reaction is complete, digest the Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA. DpnI DigestTransformHeatshock 5ul of the Dpn1-digested MEGAWHOP reaction mixture into 25ul of chemically competent E coli. | ||||||||
| Added: | ||||||||
| > > | ExampleChange the promoter for sgRNA using MegaWHOP. | |||||||
| Added: | ||||||||
| > > | Template sequence: https://benchling.com/s/g4S95i24
Primers:
 *PCR Recombinant Plasmid:
*PCR Recombinant Plasmid: 
| |||||||
| ||||||||
| Added: | ||||||||
| > > |
| |||||||
Revision 82016-04-29 - PengGeng
Megaprimer whole plasmid cloningaka MEGAWHOP cloningaka Overlap Extension PCR cloning Adapted from Bryksin AV, Matsumura I. 2010. Overlap extension PCR Cloning: a simple and reliable way to create recombinant plasmids. Biotechniques.48(6):463-5. PurposeTo insert a DNA sequence into a plasmid without restriction enzymes.Experimental Steps
Designing Primers Primers need to have two components
Primers need to have two components
PCR InsertUse stardard 25ul Phusion (or other high fidelity polymerase) protocol
PCR Recombinant PlasmidUse modified 10ul Phusion (or other high fidelity polymerase) protocol
| ||||||||
| Added: | ||||||||
| > > |
OR
Use modified 25ul Phusion protocol
| |||||||
DigestOnce the reaction is complete, digest the Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA. DpnI DigestTransformHeatshock 5ul of the Dpn1-digested MEGAWHOP reaction mixture into 25ul of chemically competent E coli.
| ||||||||
Revision 72014-07-21 - SeanLeonard
Megaprimer whole plasmid cloningaka MEGAWHOP cloningaka Overlap Extension PCR cloning Adapted from Bryksin AV, Matsumura I. 2010. Overlap extension PCR Cloning: a simple and reliable way to create recombinant plasmids. Biotechniques.48(6):463-5. PurposeTo insert a DNA sequence into a plasmid without restriction enzymes.Experimental Steps
Designing Primers Primers need to have two components
Primers need to have two components
PCR InsertUse stardard 25ul Phusion (or other high fidelity polymerase) protocol
PCR Recombinant PlasmidUse modified 10ul Phusion (or other high fidelity polymerase) protocol
Digest | ||||||||
| Changed: | ||||||||
| < < | Once the reaction is complete, digest the Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA. | |||||||
| > > | Once the reaction is complete, digest the Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA. DpnI Digest | |||||||
TransformHeatshock 5ul of the Dpn1-digested MEGAWHOP reaction mixture into 25ul of chemically competent E coli.
| ||||||||
Revision 62014-06-16 - JeffreyBarrick
Revision 52013-09-09 - JeffreyBarrick
Megaprimer whole plasmid cloningaka MEGAWHOP cloningaka Overlap Extension PCR cloning Steve Sowa 4/25/2012 Adapted from Bryksin AV, Matsumura I. 2010. Overlap extension PCR Cloning: a simple and reliable way to create recombinant plasmids. Biotechniques.48(6):463-5. PurposeTo insert a DNA sequence into a plasmid without restriction enzymes.Experimental Steps
Designing Primers Primers need to have two components
Primers need to have two components
PCR InsertUse stardard 25ul Phusion (or other high fidelity polymerase) protocol
PCR Recombinant PlasmidUse modified 10ul Phusion (or other high fidelity polymerase) protocol
DigestOnce the reaction is complete, digest the Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA.TransformHeatshock 5ul of the Dpn1-digested MEGAWHOP reaction mixture into 25ul of chemically competent E coli. -- Main.SteveSowa - 25 Apr 2012
| ||||||||
| Changed: | ||||||||
| < < |
| |||||||
| > > |
| |||||||
Revision 42012-05-01 - BrianRenda
Megaprimer whole plasmid cloningaka MEGAWHOP cloningaka Overlap Extension PCR cloning Steve Sowa 4/25/2012 Adapted from Bryksin AV, Matsumura I. 2010. Overlap extension PCR Cloning: a simple and reliable way to create recombinant plasmids. Biotechniques.48(6):463-5. PurposeTo insert a DNA sequence into a plasmid without restriction enzymes.Experimental Steps
Designing Primers Primers need to have two components
Primers need to have two components
PCR InsertUse stardard 25ul Phusion (or other high fidelity polymerase) protocol
| ||||||||
| Changed: | ||||||||
| < < | Set elongation time according to size of insert | |||||||
| > > | Set elongation time according to size of insert. | |||||||
| Changed: | ||||||||
| < < | Purify PCR products | |||||||
| > > | Purify PCR products. This can either be done through a gel extraction or by adding Dpn1 directly to the Phusion reaction mixture after PCR, digesting at 37°C for 1 hour, and then doing a standard PCR clean up. Dpn1 works efficiently in a Phusion reaction mixture. | |||||||
PCR Recombinant PlasmidUse modified 10ul Phusion (or other high fidelity polymerase) protocol
| ||||||||
| Changed: | ||||||||
| < < |
| |||||||
| > > |
| |||||||
| ||||||||
| Changed: | ||||||||
| < < | Adjust Elongation time for the length of the entire plasmid | |||||||
| > > | Adjust Elongation time for the length of the entire plasmid (90 seconds per kb). | |||||||
Digest | ||||||||
| Changed: | ||||||||
| < < | Digest Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA | |||||||
| > > | Once the reaction is complete, digest the Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNA. | |||||||
Transform | ||||||||
| Changed: | ||||||||
| < < | Electroporate the new plasmid into E coli | |||||||
| > > | Heatshock 5ul of the Dpn1-digested MEGAWHOP reaction mixture into 25ul of chemically competent E coli. | |||||||
| -- Main.SteveSowa - 25 Apr 2012 | ||||||||
| Deleted: | ||||||||
| < < | ||||||||
| ||||||||
Revision 32012-04-30 - SteveSowa
Megaprimer whole plasmid cloningaka MEGAWHOP cloningaka Overlap Extension PCR cloning Steve Sowa 4/25/2012 Adapted from Bryksin AV, Matsumura I. 2010. Overlap extension PCR Cloning: a simple and reliable way to create recombinant plasmids. Biotechniques.48(6):463-5. PurposeTo insert a DNA sequence into a plasmid without restriction enzymes.Experimental Steps
| ||||||||
| Added: | ||||||||
| > > | | |||||||
| Deleted: | ||||||||
| < < |
 | |||||||
Designing Primers Primers need to have two components
Primers need to have two components
PCR InsertUse stardard 25ul Phusion (or other high fidelity polymerase) protocol
PCR Recombinant PlasmidUse modified 10ul Phusion (or other high fidelity polymerase) protocol
DigestDigest Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNATransformElectroporate the new plasmid into E coli -- Main.SteveSowa - 25 Apr 2012 | ||||||||
| Changed: | ||||||||
| < < |
| |||||||
| > > | ||||||||
| ||||||||
| Added: | ||||||||
| > > |
| |||||||
Revision 22012-04-26 - JeffreyBarrick
| ||||||||
| Changed: | ||||||||
| < < | MEGAWHOP | |||||||
| > > | Megaprimer whole plasmid cloning | |||||||
| Added: | ||||||||
| > > | aka MEGAWHOP cloning aka Overlap Extension PCR cloning | |||||||
Steve Sowa
4/25/2012
Adapted from Bryksin AV, Matsumura I. 2010. Overlap extension PCR Cloning: a simple and reliable way to create recombinant plasmids. Biotechniques.48(6):463-5.
PurposeTo insert a DNA sequence into a plasmid without restriction enzymes.Experimental Steps

Designing Primers Primers need to have two components
Primers need to have two components
PCR InsertUse stardard 25ul Phusion (or other high fidelity polymerase) protocol
PCR Recombinant PlasmidUse modified 10ul Phusion (or other high fidelity polymerase) protocol
DigestDigest Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNATransformElectroporate the new plasmid into E coli -- Main.SteveSowa - 25 Apr 2012
| ||||||||
Revision 12012-04-25 - SteveSowa
MEGAWHOPSteve Sowa 4/25/2012 Adapted from Bryksin AV, Matsumura I. 2010. Overlap extension PCR Cloning: a simple and reliable way to create recombinant plasmids. Biotechniques.48(6):463-5.PurposeTo insert a DNA sequence into a plasmid without restriction enzymes.Experimental Steps

Designing Primers Primers need to have two components
Primers need to have two components
PCR InsertUse stardard 25ul Phusion (or other high fidelity polymerase) protocol
PCR Recombinant PlasmidUse modified 10ul Phusion (or other high fidelity polymerase) protocol
DigestDigest Recombinant Plasmid PCR product with 0.5 ul Dpn1 at 37°C for one and a half hours to remove parental DNATransformElectroporate the new plasmid into E coli -- Main.SteveSowa - 25 Apr 2012
|
View topic | History: r12 < r11 < r10 < r9 | More topic actions...