Difference: ProtocolsAcinetobacterGenomeManipulation (1 vs. 8)
Revision 82020-12-03 - IsaacGifford
Acinetobacter baylyi ADP1 Genome Manipulations | |||||||||||||||
| Added: | |||||||||||||||
| > > | Genome manipulations in Acinetobacter baylyi ADP1 can be performed without the need for exogenous recombinase expression, plasmid electroporation, or other practices used in other canonical systems such as E. coli. With just the transformation of PCR products, one can make gene knockouts, insertions, allelic replacement, and deletions. Novel DNA sequences are inserted into the ADP1 genome by recombination between flanking DNA added through ligation or overlap extension PCR and a targeted homologous region of the genome. The listed protocols use the tdk-kan selection/counter selection cassette as retrieved from the ADP1 single gene knockout project, but other selection/counter-selection cassettes (such as kan-sacB) may also be used. The Barrick Lab primarily uses the Golden Transformation method linked below for constructing modified ADP1 strains which is fast and nearly scarless. | ||||||||||||||
| Changed: | |||||||||||||||
| < < | Overview | ||||||||||||||
| > > | Protocols for ADP1 genome manipulations include: | ||||||||||||||
| Added: | |||||||||||||||
| > > |
| ||||||||||||||
| Changed: | |||||||||||||||
| < < | Genome manipulations in Acinetobacter baylyi ADP1 can be performed without the need for exogenous recombinase expression, plasmid electroporation, or other practices used in other canonical systems such as E. coli. With just the transformation of PCR products, one can make gene knockouts, insertions, allelic replacement, and deletions. The listed protocols use the tdk-kan selection/counter selection cassette as retrieved from the ADP1 single gene knockout project, but other selection/counter-selection cassettes (such as kan-sacB) may also be used. Our general strategy is to first construct our transforming constructs with targeted homology to the genome using a 3 piece overlap PCR and then (for all procedures minus simple knock outs) rescuing the insertion of that cassette into the genome with the desired construct using the tdk counter-selection. | ||||||||||||||
| > > | DNA for genome modifications can be transformed into ADP1 using either overnight culture transformations or "in plate" or "puddle" transformations. | ||||||||||||||
| Deleted: | |||||||||||||||
| < < |
Overlap PCR Construct GenerationThe following is a standard procedure designing and constructing ADP1 genome manipulation constructs using overlap PCR.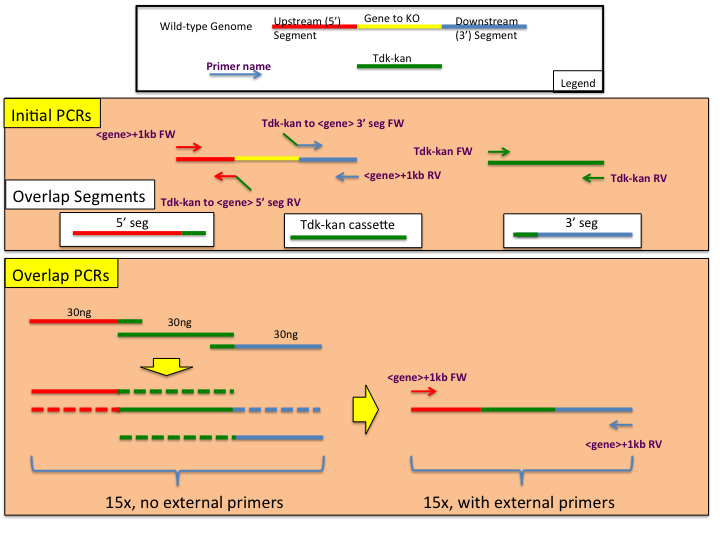
The Design of Overlap PCR Primers and ConstructsIn order to ensure a successful assembly of transforming constructs, particular care should be made when designing the appropriate primers. A total of 6 primers are needed (see Initial PCRs panel in the provided figure), two of which will add homology of the tdk-kan cassette to the upstream flanking region (5' seg) and downstream flanking region (3' seg). The following guidelines for primer construction should be followed:
Overlap PCR ReactionsInitial PCR of 5' SegmentIn a PCR tube, combine the following:
Initial PCR of tdk-kan SegmentIn a PCR tube, combine the following:
Initial PCR of 3' SegmentIn a PCR tube, combine the following:
Overlap PCR ReactionIn a PCR tube, combine the following:
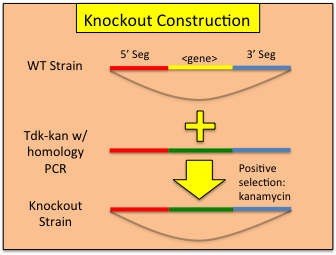
Constructing Gene Knockouts (or interruptions) in A. baylyi ADP1A collection of single gene knockouts has been published ( http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2290942/ ) is currently available at http://www.genoscope.cns.fr/spip/Strain-request-for-mutants-of,749.html . However, if you wish to construct your own knockouts, use the following work flow:
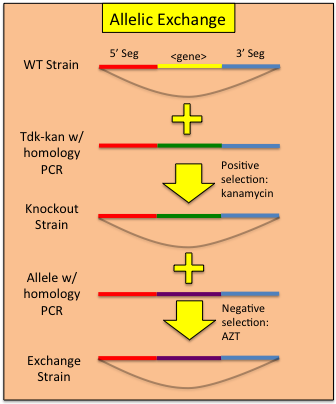
Constructing Allelic ReplacementsOnce one has replaced a gene in the ADP1 genome with the tdk-kan cassette, a mutated allele can be used to 'rescue' the bacteria from the cassette via transformation and plating on AZT. Note: zidovudine is the pharmaceutical name for AZT and can be ordered at far less cost. In our hands, we find zidovudine to be just as effective in counter-selections and should be used at the same concentration as AZT. Combining a targeted transformation of the tdk-kan cassette with an allelic rescue effectively allows a 2 step process for replicating any mutant allele in any competent ADP1 genomic background. To perform allelic rescue:
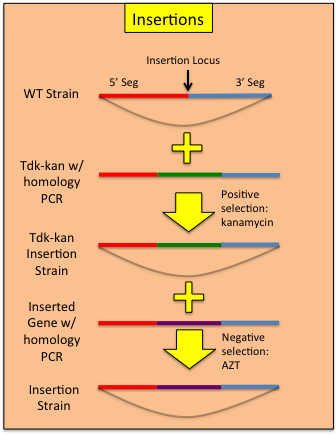
Constructing InsertionsUsing a modified allelic exchange procedure, genetic constructs can be added to the ADP1 genome without the need to maintain a selective marker.
ContributorsBrian Renda, Gabriel Suarez | ||||||||||||||
| |||||||||||||||
Revision 72016-04-14 - GabrielSuarez
Acinetobacter baylyi ADP1 Genome ManipulationsOverview | |||||||||||||||
| Changed: | |||||||||||||||
| < < | Genome manipulations in Acinetobacter baylyi ADP1 can be performed without the need for exogenous recombinase expression, plasmid electroporation, or other practices used in other canonical systems such as E. coli. With just the transformation of PCR products, one can make gene knockouts, insertions, allelic replacement, and deletions. The listed protocols use the tdk-kan selection/counter selection cassette as retrieved from the ADP1 single gene knockout project, but other selection/counter-selection cassettes (such as kan-sacB) may also be used. Our general strategy is to first construct our transforming constructs with targeted homology to the genome using a 3 piece overlap PCR and then (for all procedures minus simple knock outs) rescuing the insertion of that cassette into the genome with the desired construct using the tdk counter-selection. | ||||||||||||||
| > > | Genome manipulations in Acinetobacter baylyi ADP1 can be performed without the need for exogenous recombinase expression, plasmid electroporation, or other practices used in other canonical systems such as E. coli. With just the transformation of PCR products, one can make gene knockouts, insertions, allelic replacement, and deletions. The listed protocols use the tdk-kan selection/counter selection cassette as retrieved from the ADP1 single gene knockout project, but other selection/counter-selection cassettes (such as kan-sacB) may also be used. Our general strategy is to first construct our transforming constructs with targeted homology to the genome using a 3 piece overlap PCR and then (for all procedures minus simple knock outs) rescuing the insertion of that cassette into the genome with the desired construct using the tdk counter-selection. | ||||||||||||||
Overlap PCR Construct GenerationThe following is a standard procedure designing and constructing ADP1 genome manipulation constructs using overlap PCR.
The Design of Overlap PCR Primers and Constructs | |||||||||||||||
| Changed: | |||||||||||||||
| < < | In order to ensure a successful assembly of transforming constructs, particular care should be made when designing the appropriate primers. A total of 6 primers are needed (see Initial PCRs panel in the provided figure), two of which will add homology of the tdk-kan cassette to the upstream flanking region (5' seg) and downstream flanking region (3' seg). The following guidelines for primer construction should be followed: | ||||||||||||||
| > > | In order to ensure a successful assembly of transforming constructs, particular care should be made when designing the appropriate primers. A total of 6 primers are needed (see Initial PCRs panel in the provided figure), two of which will add homology of the tdk-kan cassette to the upstream flanking region (5' seg) and downstream flanking region (3' seg). The following guidelines for primer construction should be followed: | ||||||||||||||
Overlap PCR ReactionsInitial PCR of 5' SegmentIn a PCR tube, combine the following:
Initial PCR of tdk-kan SegmentIn a PCR tube, combine the following:
Initial PCR of 3' SegmentIn a PCR tube, combine the following:
Overlap PCR ReactionIn a PCR tube, combine the following:
Constructing Gene Knockouts (or interruptions) in A. baylyi ADP1A collection of single gene knockouts has been published ( http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2290942/ ) is currently available at http://www.genoscope.cns.fr/spip/Strain-request-for-mutants-of,749.html . However, if you wish to construct your own knockouts, use the following work flow:
Constructing Allelic ReplacementsOnce one has replaced a gene in the ADP1 genome with the tdk-kan cassette, a mutated allele can be used to 'rescue' the bacteria from the cassette via transformation and plating on AZT. Note: zidovudine is the pharmaceutical name for AZT and can be ordered at far less cost. In our hands, we find zidovudine to be just as effective in counter-selections and should be used at the same concentration as AZT. Combining a targeted transformation of the tdk-kan cassette with an allelic rescue effectively allows a 2 step process for replicating any mutant allele in any competent ADP1 genomic background. To perform allelic rescue:
Constructing InsertionsUsing a modified allelic exchange procedure, genetic constructs can be added to the ADP1 genome without the need to maintain a selective marker.

ContributorsBrian Renda, Gabriel Suarez
| |||||||||||||||
Revision 62016-04-08 - JeffreyBarrick
Acinetobacter baylyi ADP1 Genome ManipulationsOverviewGenome manipulations in Acinetobacter baylyi ADP1 can be performed without the need for exogenous recombinase expression, plasmid electroporation, or other practices used in other canonical systems such as E. coli. With just the transformation of PCR products, one can make gene knockouts, insertions, allelic replacement, and deletions. The listed protocols use the tdk-kan selection/counter selection cassette as retrieved from the ADP1 single gene knockout project, but other selection/counter-selection cassettes (such as kan-sacB) may also be used. Our general strategy is to first construct our transforming constructs with targeted homology to the genome using a 3 piece overlap PCR and then (for all procedures minus simple knock outs) rescuing the insertion of that cassette into the genome with the desired construct using the tdk counter-selection.Overlap PCR Construct GenerationThe following is a standard procedure designing and constructing ADP1 genome manipulation constructs using overlap PCR.
The Design of Overlap PCR Primers and ConstructsIn order to ensure a successful assembly of transforming constructs, particular care should be made when designing the appropriate primers. A total of 6 primers are needed (see Initial PCRs panel in the provided figure), two of which will add homology of the tdk-kan cassette to the upstream flanking region (5' seg) and downstream flanking region (3' seg). The following guidelines for primer construction should be followed:
Overlap PCR ReactionsInitial PCR of 5' SegmentIn a PCR tube, combine the following:
Initial PCR of tdk-kan SegmentIn a PCR tube, combine the following:
Initial PCR of 3' SegmentIn a PCR tube, combine the following:
Overlap PCR ReactionIn a PCR tube, combine the following:
Constructing Gene Knockouts (or interruptions) in A. baylyi ADP1A collection of single gene knockouts has been published ( http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2290942/ ) is currently available at http://www.genoscope.cns.fr/spip/Strain-request-for-mutants-of,749.html . However, if you wish to construct your own knockouts, use the following work flow:
Constructing Allelic ReplacementsOnce one has replaced a gene in the ADP1 genome with the tdk-kan cassette, a mutated allele can be used to 'rescue' the bacteria from the cassette via transformation and plating on AZT. Note: zidovudine is the pharmaceutical name for AZT and can be ordered at far less cost. In our hands, we find zidovudine to be just as effective in counter-selections and should be used at the same concentration as AZT. Combining a targeted transformation of the tdk-kan cassette with an allelic rescue effectively allows a 2 step process for replicating any mutant allele in any competent ADP1 genomic background. To perform allelic rescue:
Constructing InsertionsUsing a modified allelic exchange procedure, genetic constructs can be added to the ADP1 genome without the need to maintain a selective marker.
 | |||||||||||||||
| Changed: | |||||||||||||||
| < < | -- Main.BrianRenda - 13 Jun 2013 | ||||||||||||||
| > > | Contributors | ||||||||||||||
| Added: | |||||||||||||||
| > > | Brian Renda, Gabriel Suarez | ||||||||||||||
| Changed: | |||||||||||||||
| < < |
| ||||||||||||||
| > > |
| ||||||||||||||
Revision 52016-04-07 - GabrielSuarez
Acinetobacter baylyi ADP1 Genome ManipulationsOverviewGenome manipulations in Acinetobacter baylyi ADP1 can be performed without the need for exogenous recombinase expression, plasmid electroporation, or other practices used in other canonical systems such as E. coli. With just the transformation of PCR products, one can make gene knockouts, insertions, allelic replacement, and deletions. The listed protocols use the tdk-kan selection/counter selection cassette as retrieved from the ADP1 single gene knockout project, but other selection/counter-selection cassettes (such as kan-sacB) may also be used. Our general strategy is to first construct our transforming constructs with targeted homology to the genome using a 3 piece overlap PCR and then (for all procedures minus simple knock outs) rescuing the insertion of that cassette into the genome with the desired construct using the tdk counter-selection.Overlap PCR Construct GenerationThe following is a standard procedure designing and constructing ADP1 genome manipulation constructs using overlap PCR. | |||||||||||||||
| Changed: | |||||||||||||||
| < < |  | ||||||||||||||
| > > |  | ||||||||||||||
The Design of Overlap PCR Primers and ConstructsIn order to ensure a successful assembly of transforming constructs, particular care should be made when designing the appropriate primers. A total of 6 primers are needed (see Initial PCRs panel in the provided figure), two of which will add homology of the tdk-kan cassette to the upstream flanking region (5' seg) and downstream flanking region (3' seg). The following guidelines for primer construction should be followed:
Overlap PCR ReactionsInitial PCR of 5' SegmentIn a PCR tube, combine the following:
Initial PCR of tdk-kan SegmentIn a PCR tube, combine the following:
Initial PCR of 3' SegmentIn a PCR tube, combine the following:
Overlap PCR ReactionIn a PCR tube, combine the following:
Constructing Gene Knockouts (or interruptions) in A. baylyi ADP1A collection of single gene knockouts has been published ( http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2290942/ ) is currently available at http://www.genoscope.cns.fr/spip/Strain-request-for-mutants-of,749.html . However, if you wish to construct your own knockouts, use the following work flow:
Constructing Allelic ReplacementsOnce one has replaced a gene in the ADP1 genome with the tdk-kan cassette, a mutated allele can be used to 'rescue' the bacteria from the cassette via transformation and plating on AZT. Note: zidovudine is the pharmaceutical name for AZT and can be ordered at far less cost. In our hands, we find zidovudine to be just as effective in counter-selections and should be used at the same concentration as AZT. Combining a targeted transformation of the tdk-kan cassette with an allelic rescue effectively allows a 2 step process for replicating any mutant allele in any competent ADP1 genomic background. To perform allelic rescue:
Constructing InsertionsUsing a modified allelic exchange procedure, genetic constructs can be added to the ADP1 genome without the need to maintain a selective marker.
 -- Main.BrianRenda - 13 Jun 2013
-- Main.BrianRenda - 13 Jun 2013
| |||||||||||||||
Revision 42016-04-07 - BrianRenda
| |||||||||||||||
| Changed: | |||||||||||||||
| < < | Acinetobacter baylyi ADP1 Genome Manipulations | ||||||||||||||
| > > | Acinetobacter baylyi ADP1 Genome Manipulations | ||||||||||||||
Overview | |||||||||||||||
| Changed: | |||||||||||||||
| < < | Genome manipulations in Acinetobacter baylyi ADP1 (AB) can be performed without the need for exogenous recombinase expression, plasmid electroporation, or other practices used in other canonical systems such as E. coli. With just the transformation of PCR products, one can make gene knockouts, insertions, allelic replacement, and deletions. The listed protocols use the tdk-kan selection/counter selection cassette as retrieved from the AB single gene knockout project, but other selection/counter-selection cassettes (such as kan-sacB) may also be used. Our general strategy is to first construct our transforming constructs with targeted homology to the genome using a 3 piece overlap PCR and then (for all procedures minus simple knock outs) rescuing the insertion of that cassette into the genome with the desired construct using the tdk counter-selection. | ||||||||||||||
| > > | Genome manipulations in Acinetobacter baylyi ADP1 can be performed without the need for exogenous recombinase expression, plasmid electroporation, or other practices used in other canonical systems such as E. coli. With just the transformation of PCR products, one can make gene knockouts, insertions, allelic replacement, and deletions. The listed protocols use the tdk-kan selection/counter selection cassette as retrieved from the ADP1 single gene knockout project, but other selection/counter-selection cassettes (such as kan-sacB) may also be used. Our general strategy is to first construct our transforming constructs with targeted homology to the genome using a 3 piece overlap PCR and then (for all procedures minus simple knock outs) rescuing the insertion of that cassette into the genome with the desired construct using the tdk counter-selection. | ||||||||||||||
Overlap PCR Construct Generation | |||||||||||||||
| Changed: | |||||||||||||||
| < < | The following is a standard procedure designing and constructing AB genome manipulation constructs using overlap PCR. | ||||||||||||||
| > > | The following is a standard procedure designing and constructing ADP1 genome manipulation constructs using overlap PCR. | ||||||||||||||

The Design of Overlap PCR Primers and ConstructsIn order to ensure a successful assembly of transforming constructs, particular care should be made when designing the appropriate primers. A total of 6 primers are needed (see Initial PCRs panel in the provided figure), two of which will add homology of the tdk-kan cassette to the upstream flanking region (5' seg) and downstream flanking region (3' seg). The following guidelines for primer construction should be followed:
| |||||||||||||||
| Changed: | |||||||||||||||
| < < |
| ||||||||||||||
| > > |
| ||||||||||||||
| |||||||||||||||
| Changed: | |||||||||||||||
| < < | For the design of the constructs themselves, flanking regions should be 500-1000bp long to facilitate efficient transformation into the AB genome. We generally use 1kb on each side to obtain a high efficiency of transformation. | ||||||||||||||
| > > | For the design of the constructs themselves, flanking regions should be 500-1000bp long to facilitate efficient transformation into the ADP1 genome. We generally use 1kb on each side to obtain a high efficiency of transformation. | ||||||||||||||
Overlap PCR ReactionsInitial PCR of 5' SegmentIn a PCR tube, combine the following:
| |||||||||||||||
| Changed: | |||||||||||||||
| < < |
| ||||||||||||||
| > > |
| ||||||||||||||
| |||||||||||||||
| Changed: | |||||||||||||||
| < < | After the reaction, add 1uL of 20,000U/mL Dpn1 restriction enzyme and incubate at 37C for 1 hour. This will digest any background AB genomic DNA template. Once the digestion is completed, run a 5uL aliquot of the reaction on an agarose gel to check for product formation and purify the remaining 45uL reaction with a PCR cleanup kit. Do not elute in buffer containing EDTA. Quantify the DNA concentration and keep in the freezer until ready to perform the overlap PCR. | ||||||||||||||
| > > | After the reaction, add 1uL of 20,000U/mL Dpn1 restriction enzyme and incubate at 37C for 1 hour. This will digest any background ADP1 genomic DNA template. Once the digestion is completed, run a 5uL aliquot of the reaction on an agarose gel to check for product formation and purify the remaining 45uL reaction with a PCR cleanup kit. Do not elute in buffer containing EDTA. Quantify the DNA concentration and keep in the freezer until ready to perform the overlap PCR. | ||||||||||||||
Initial PCR of tdk-kan SegmentIn a PCR tube, combine the following:
| |||||||||||||||
| Changed: | |||||||||||||||
| < < |
| ||||||||||||||
| > > |
| ||||||||||||||
| |||||||||||||||
| Changed: | |||||||||||||||
| < < | After the reaction, add 1uL of 20,000U/mL Dpn1 restriction enzyme and incubate at 37C for 1 hour. This will digest any background AB genomic DNA template. Once the digestion is completed, run a 5uL aliquot of the reaction on an agarose gel to check for product formation and purify the remaining 45uL reaction with a PCR cleanup kit. Do not elute in buffer containing EDTA. Quantify the DNA concentration and keep in the freezer until ready to perform the overlap PCR. | ||||||||||||||
| > > | After the reaction, add 1uL of 20,000U/mL Dpn1 restriction enzyme and incubate at 37C for 1 hour. This will digest any background ADP1 genomic DNA template. Once the digestion is completed, run a 5uL aliquot of the reaction on an agarose gel to check for product formation and purify the remaining 45uL reaction with a PCR cleanup kit. Do not elute in buffer containing EDTA. Quantify the DNA concentration and keep in the freezer until ready to perform the overlap PCR. | ||||||||||||||
Initial PCR of 3' SegmentIn a PCR tube, combine the following:
| |||||||||||||||
| Changed: | |||||||||||||||
| < < |
| ||||||||||||||
| > > |
| ||||||||||||||
| |||||||||||||||
| Changed: | |||||||||||||||
| < < | After the reaction, add 1uL of 20,000U/mL Dpn1 restriction enzyme and incubate at 37C for 1 hour. This will digest any background AB genomic DNA template. Once the digestion is completed, run a 5uL aliquot of the reaction on an agarose gel to check for product formation and purify the remaining 45uL reaction with a PCR cleanup kit. Do not elute in buffer containing EDTA. Quantify the DNA concentration and keep in the freezer until ready to perform the overlap PCR. | ||||||||||||||
| > > | After the reaction, add 1uL of 20,000U/mL Dpn1 restriction enzyme and incubate at 37C for 1 hour. This will digest any background ADP1 genomic DNA template. Once the digestion is completed, run a 5uL aliquot of the reaction on an agarose gel to check for product formation and purify the remaining 45uL reaction with a PCR cleanup kit. Do not elute in buffer containing EDTA. Quantify the DNA concentration and keep in the freezer until ready to perform the overlap PCR. | ||||||||||||||
Overlap PCR ReactionIn a PCR tube, combine the following:
| |||||||||||||||
| Changed: | |||||||||||||||
| < < | Run this reaction for another 15 cycles with the appropriate annealing temperature. Once complete, run a 5uL aliquot of the reaction on a gel to check for the assembly of your 3.8kb product. This product is ready for transformation into AB. | ||||||||||||||
| > > | Run this reaction for another 15 cycles with the appropriate annealing temperature. Once complete, run a 5uL aliquot of the reaction on a gel to check for the assembly of your 3.8kb product. This product is ready for transformation into ADP1. | ||||||||||||||
| Changed: | |||||||||||||||
| < < | Constructing Gene Knockouts (or interruptions) in A. baylyi ADP1 | ||||||||||||||
| > > | Constructing Gene Knockouts (or interruptions) in A. baylyi ADP1 | ||||||||||||||
A collection of single gene knockouts has been published ( http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2290942/ ) is currently available at http://www.genoscope.cns.fr/spip/Strain-request-for-mutants-of,749.html . However, if you wish to construct your own knockouts, use the following work flow:
| |||||||||||||||
| Changed: | |||||||||||||||
| < < |
| ||||||||||||||
| > > |
| ||||||||||||||
Constructing Allelic Replacements | |||||||||||||||
| Changed: | |||||||||||||||
| < < | Once one has replaced a gene in the AB genome with the tdk-kan cassette, a mutated allele can be used to 'rescue' the bacteria from the cassette via transformation and plating on AZT. Combining a targeted transformation of the tdk-kan cassette with an allelic rescue effectively allows a 2 step process for replicating any mutant allele in any competent AB genomic background. To perform allelic rescue: | ||||||||||||||
| > > | Once one has replaced a gene in the ADP1 genome with the tdk-kan cassette, a mutated allele can be used to 'rescue' the bacteria from the cassette via transformation and plating on AZT. Note: zidovudine is the pharmaceutical name for AZT and can be ordered at far less cost. In our hands, we find zidovudine to be just as effective in counter-selections and should be used at the same concentration as AZT. Combining a targeted transformation of the tdk-kan cassette with an allelic rescue effectively allows a 2 step process for replicating any mutant allele in any competent ADP1 genomic background. To perform allelic rescue: | ||||||||||||||
| Changed: | |||||||||||||||
| < < |
| ||||||||||||||
| > > |
| ||||||||||||||
Constructing Insertions | |||||||||||||||
| Changed: | |||||||||||||||
| < < | Using a modified allelic exchange procedure, genetic constructs can be added to the AB genome without the need to maintain a selective marker. | ||||||||||||||
| > > | Using a modified allelic exchange procedure, genetic constructs can be added to the ADP1 genome without the need to maintain a selective marker. | ||||||||||||||
 -- Main.BrianRenda - 13 Jun 2013
-- Main.BrianRenda - 13 Jun 2013
| |||||||||||||||
Revision 32013-06-14 - BrianRenda
Acinetobacter baylyi ADP1 Genome ManipulationsOverviewGenome manipulations in Acinetobacter baylyi ADP1 (AB) can be performed without the need for exogenous recombinase expression, plasmid electroporation, or other practices used in other canonical systems such as E. coli. With just the transformation of PCR products, one can make gene knockouts, insertions, allelic replacement, and deletions. The listed protocols use the tdk-kan selection/counter selection cassette as retrieved from the AB single gene knockout project, but other selection/counter-selection cassettes (such as kan-sacB) may also be used. Our general strategy is to first construct our transforming constructs with targeted homology to the genome using a 3 piece overlap PCR and then (for all procedures minus simple knock outs) rescuing the insertion of that cassette into the genome with the desired construct using the tdk counter-selection.Overlap PCR Construct GenerationThe following is a standard procedure designing and constructing AB genome manipulation constructs using overlap PCR.
The Design of Overlap PCR Primers and ConstructsIn order to ensure a successful assembly of transforming constructs, particular care should be made when designing the appropriate primers. A total of 6 primers are needed (see Initial PCRs panel in the provided figure), two of which will add homology of the tdk-kan cassette to the upstream flanking region (5' seg) and downstream flanking region (3' seg). The following guidelines for primer construction should be followed:
| |||||||||||||||
| Changed: | |||||||||||||||
| < < | For the design of the constructs themselves, flanking regions should be 500-1000bp long to facilitate efficient transformation into the AB genome. We generally use 1kb on each side due to the high efficiency of transformation. | ||||||||||||||
| > > | For the design of the constructs themselves, flanking regions should be 500-1000bp long to facilitate efficient transformation into the AB genome. We generally use 1kb on each side to obtain a high efficiency of transformation. | ||||||||||||||
Overlap PCR ReactionsInitial PCR of 5' SegmentIn a PCR tube, combine the following:
| |||||||||||||||
| Changed: | |||||||||||||||
| < < | Run with a standard phusion PCR protocol with the appropriate annealing temperature, 45 second 72C elongation, and 29 repetitions. | ||||||||||||||
| > > | Run with a standard phusion PCR protocol with the appropriate annealing temperature, 45 second 72C elongation (the literature suggests 15-30 seconds per kb, but I tend to overshoot elongation times), and 29 repetitions. | ||||||||||||||
After the reaction, add 1uL of 20,000U/mL Dpn1 restriction enzyme and incubate at 37C for 1 hour. This will digest any background AB genomic DNA template. Once the digestion is completed, run a 5uL aliquot of the reaction on an agarose gel to check for product formation and purify the remaining 45uL reaction with a PCR cleanup kit. Do not elute in buffer containing EDTA. Quantify the DNA concentration and keep in the freezer until ready to perform the overlap PCR.
Initial PCR of tdk-kan SegmentIn a PCR tube, combine the following:
Initial PCR of 3' SegmentIn a PCR tube, combine the following:
Overlap PCR ReactionIn a PCR tube, combine the following:
Constructing Gene Knockouts (or interruptions) in A. baylyi ADP1A collection of single gene knockouts has been published ( http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2290942/ ) is currently available at http://www.genoscope.cns.fr/spip/Strain-request-for-mutants-of,749.html . However, if you wish to construct your own knockouts, use the following work flow:
Constructing Allelic ReplacementsOnce one has replaced a gene in the AB genome with the tdk-kan cassette, a mutated allele can be used to 'rescue' the bacteria from the cassette via transformation and plating on AZT. Combining a targeted transformation of the tdk-kan cassette with an allelic rescue effectively allows a 2 step process for replicating any mutant allele in any competent AB genomic background. To perform allelic rescue:
Constructing InsertionsUsing a modified allelic exchange procedure, genetic constructs can be added to the AB genome without the need to maintain a selective marker.
 -- Main.BrianRenda - 13 Jun 2013
-- Main.BrianRenda - 13 Jun 2013
| |||||||||||||||
Revision 22013-06-13 - BrianRenda
| |||||||||||||||
| Changed: | |||||||||||||||
| < < | Acinetobacter baylyi ADP1 Genome Manipulations - Note: Protocol Construction In Progress | ||||||||||||||
| > > | Acinetobacter baylyi ADP1 Genome Manipulations | ||||||||||||||
| Deleted: | |||||||||||||||
| < < | |||||||||||||||
OverviewGenome manipulations in Acinetobacter baylyi ADP1 (AB) can be performed without the need for exogenous recombinase expression, plasmid electroporation, or other practices used in other canonical systems such as E. coli. With just the transformation of PCR products, one can make gene knockouts, insertions, allelic replacement, and deletions. The listed protocols use the tdk-kan selection/counter selection cassette as retrieved from the AB single gene knockout project, but other selection/counter-selection cassettes (such as kan-sacB) may also be used. Our general strategy is to first construct our transforming constructs with targeted homology to the genome using a 3 piece overlap PCR and then (for all procedures minus simple knock outs) rescuing the insertion of that cassette into the genome with the desired construct using the tdk counter-selection.Overlap PCR Construct Generation | |||||||||||||||
| Changed: | |||||||||||||||
| < < | The following is a standard procedure designing and constructing AB genome manipulation constructs using overlap PCR. | ||||||||||||||
| > > | The following is a standard procedure designing and constructing AB genome manipulation constructs using overlap PCR.  | ||||||||||||||
The Design of Overlap PCR Primers and Constructs | |||||||||||||||
| Changed: | |||||||||||||||
| < < | In order to ensure a successful assembly of transforming constructs, particular care should be made when designing the appropriate primers. | ||||||||||||||
| > > | In order to ensure a successful assembly of transforming constructs, particular care should be made when designing the appropriate primers. A total of 6 primers are needed (see Initial PCRs panel in the provided figure), two of which will add homology of the tdk-kan cassette to the upstream flanking region (5' seg) and downstream flanking region (3' seg). The following guidelines for primer construction should be followed: | ||||||||||||||
| Changed: | |||||||||||||||
| < < | | ||||||||||||||
| > > |
| ||||||||||||||
| Changed: | |||||||||||||||
| < < | Notes/Considerations in AB transformations | ||||||||||||||
| > > | For the design of the constructs themselves, flanking regions should be 500-1000bp long to facilitate efficient transformation into the AB genome. We generally use 1kb on each side due to the high efficiency of transformation. | ||||||||||||||
| Deleted: | |||||||||||||||
| < < |
| ||||||||||||||
| Added: | |||||||||||||||
| > > | Overlap PCR ReactionsInitial PCR of 5' SegmentIn a PCR tube, combine the following:
Initial PCR of tdk-kan SegmentIn a PCR tube, combine the following:
Initial PCR of 3' SegmentIn a PCR tube, combine the following:
Overlap PCR ReactionIn a PCR tube, combine the following:
Constructing Gene Knockouts (or interruptions) in A. baylyi ADP1A collection of single gene knockouts has been published ( http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2290942/ ) is currently available at http://www.genoscope.cns.fr/spip/Strain-request-for-mutants-of,749.html . However, if you wish to construct your own knockouts, use the following work flow:
Constructing Allelic ReplacementsOnce one has replaced a gene in the AB genome with the tdk-kan cassette, a mutated allele can be used to 'rescue' the bacteria from the cassette via transformation and plating on AZT. Combining a targeted transformation of the tdk-kan cassette with an allelic rescue effectively allows a 2 step process for replicating any mutant allele in any competent AB genomic background. To perform allelic rescue:
Constructing InsertionsUsing a modified allelic exchange procedure, genetic constructs can be added to the AB genome without the need to maintain a selective marker.
 | ||||||||||||||
| -- Main.BrianRenda - 13 Jun 2013 | |||||||||||||||
| Added: | |||||||||||||||
| > > |
| ||||||||||||||
Revision 12013-06-13 - BrianRenda
View topic | History: r8 < r7 < r6 < r5 | More topic actions...